Determination of the cleanliness of medical devices
The quality and safety requirements for medical devices are becoming increasingly stringent. For this reason, the cleanliness of medical devices is being examined more closely.
With its many years of experience in analytical chemistry and certification of medical devices, RMS offers its customers the planning, execution, and documentation of cleanliness determination. If possible, the analyses are carried out in our own laboratories, otherwise we rely on competent laboratories, to which we can forward analyses in agreement with the customer.
Procedure
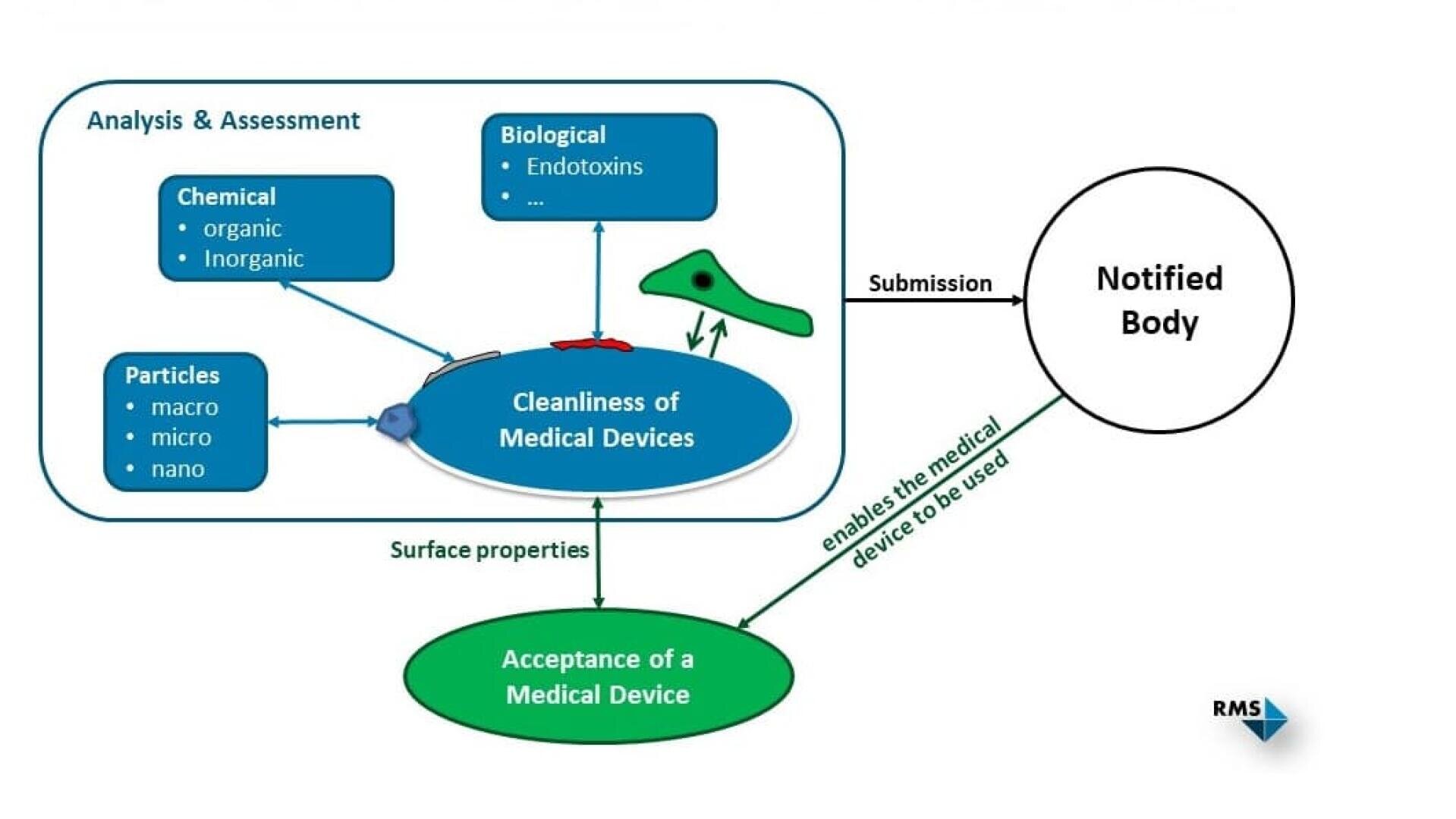
In order to demonstrate the cleanliness of a medical device, the degree of contamination must be below specified or previously defined acceptance criteria. The contaminations to be considered can be divided into the following classes:
- Chemical (inorganic and organic)
- Biological (e.g. bioburden, endotoxins)
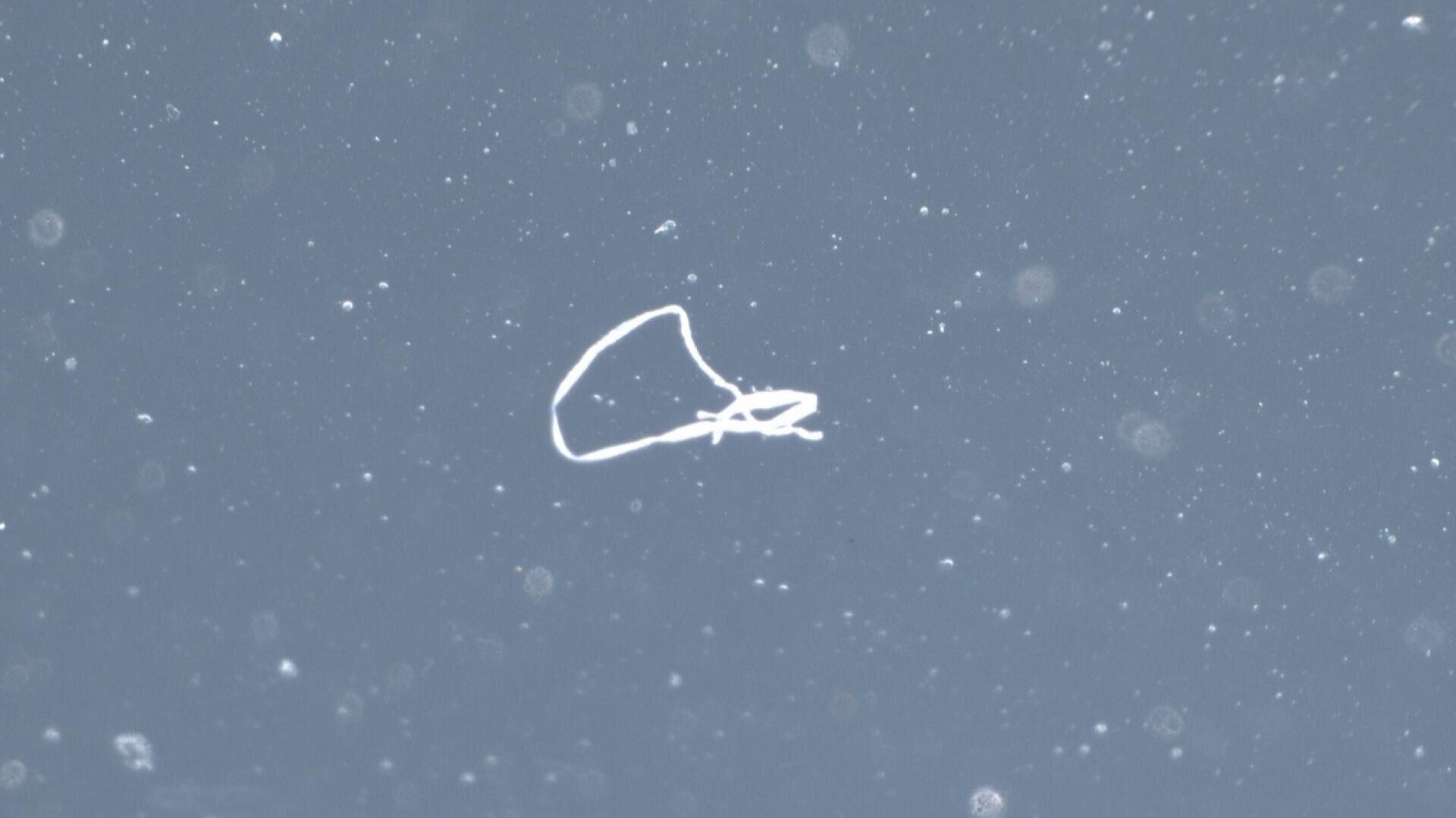
- Particulate contaminants
If there is no product-specific standard that specifies acceptance criteria for cleanliness, these must be defined for the different contamination classes and plausibly justified by the manufacturer. Related standards or clinical experience can be used to assist in this process. According to ISO 19227:2018, an acceptable level of residual contamination should not affect the function or biocompatibility (ISO 10993 series of standards) of a medical device.
The test methods can be divided into direct (direct measurement on medical device) and indirect (removal of residues for testing) methods. A direct analysis (e.g. XPS, FT-IR, EDX) has the advantage that the sample preparation, which can strongly influence the results, is reduced to a minimum. On the other hand, a direct and therefore local analysis is not necessarily representative for the entire medical device. If we want to analyse the cleanliness of the entire medical device (e.g. total amount of particles), an extraction is performed before the analysis and the extract is examined. Organic residues can be determined e.g. by FT-IR, HPLC, GC-MS or TOC, while inorganic residues can be analysed by ICP-MS in the ultra-trace range. To classify and quantify particles, the extracts must first be filtered and the filter is then examined optically or by SEM. The chemical composition of the particles can be determined by FT-IR or EDX analysis.
How complete and correct the results of such an analysis are depends strongly on how suitable the selected extraction conditions (e.g. extraction medium, temperature, agitation) are for recovering the contaminants present from the product surface. The selection of the extraction conditions is carried out according to normative specifications (e.g. ISO 10993-12:2012) and is specifically adapted to the medical device to be tested.
For the biological testing, we collaborate with reliable and competent laboratories, to which we can forward analyses in agreement with the customer.
If contaminants are detected, they must be quantified and their toxicity assessed. For this purpose, we offer literature-based toxicological risk assessments according to ISO 19993-1:2018 and ISO 10993-17:2002.
Discuss your questions with us!
We are happy to advise you. Your contact for cleanliness determination: Dr. Marc Bohner.