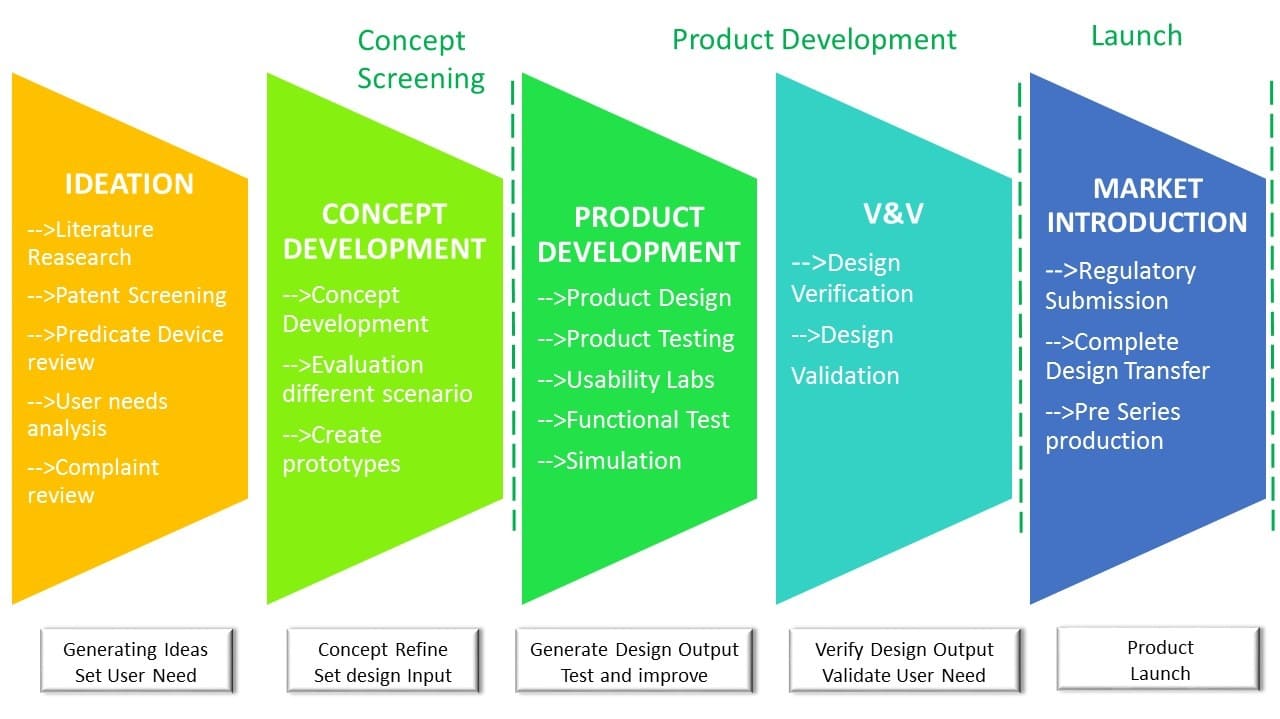
We provide support to our clients in getting trought all the product development stages.
From ideation to final market introduction.
We are ISO 13485 certified for Medical Device Design, Development and all related activities.
Medical Device Design and Development - We provide support to our clients in getting trought all the product development stages:
From ideation to final market introduction. We can support and guide trought all the develoment process. We are ISO 13485 certified for Medical Device Design, Development and all related activities.
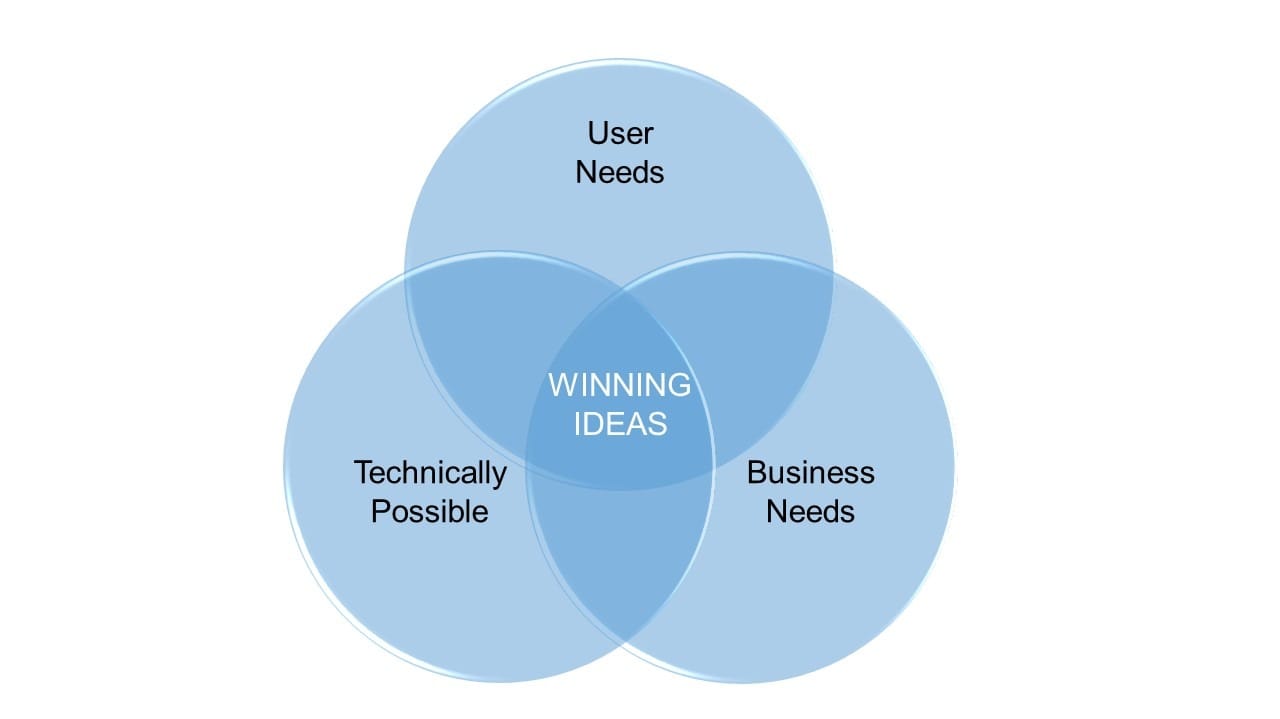
Defining and addressing the User Needs, matching Technical possibilities to Business needs find best solutions and finally develop a successful product.
We use all necessary tool to develop a succesfull device from concept phase to final design:
- 3D modelers to define the design of the product
- 2D CAD systems to allow and facilitate industrialization,
- Reverse Engineering to reproduce design elements from other components,
- Rapid Prototyping to quickly get to the prototypes of the product to be challenged and verified
- Finite Element Simulations (FEM) to optimize the design according to the operating conditions
We have huge experience in medical device development and design with particular focus to following fields:
- Orthopaedics
- Traumatology
- Neurosurgery
- Cardiovascular
- Surgical Instruments
- Combination products
- Dental device
We not only design and develop the device but also help you getting on the market with our Regulatory, Quality and Validation Support Services.
Regulatory Support
We work for our client in order to get their product on the market efficiently and effectivly. We provide a full range of regulatory support service, with particular focus to:
- Technical File / Design Dossier
- Design Control
- DHF Design History File
- Risk Management Process
- Clinical Evaluation
Quality Support
- Quality System Support
- SOP – IOP
- VMP IQ/OQ/PQ Validation
- Clean Room Design and Validation
- ISO 13485 Auditing Service