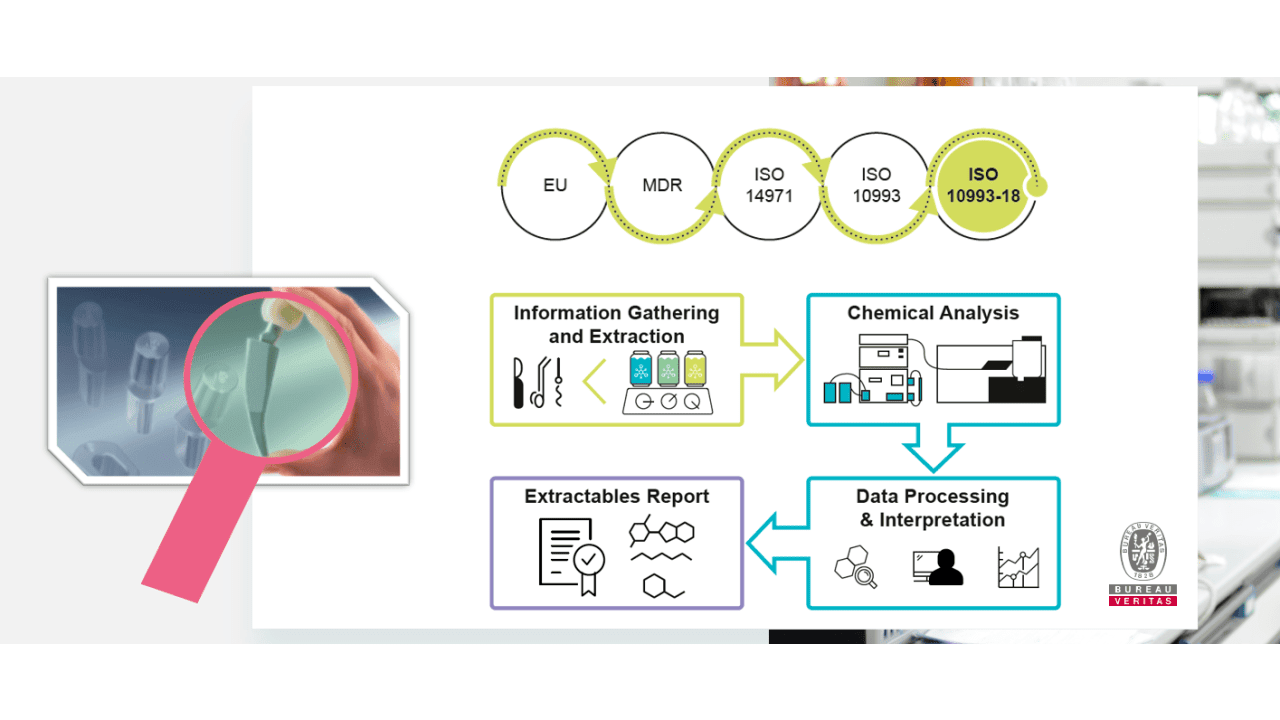
The requirements for an ISO 14971 risk management in the biological assessment of medical devices have significantly changed.
We would be pleased to support you as experts in ISO 10993-18: "chemical characterization" in meeting the new challenges.
For the overall biological evaluation of a medical device the chemical characterization has been prioritized. In the past, in vitro and in vivo testing were the golden standard that has been changed by the alignment of ISO 10993 with MDR requirements
Our state-of-the-art equipment accompanied with a highly skilled team allows us to identify and quantify an extraordinary large number/ big scope of compounds.
In doing so, we reduce your uncertainties to a minimum, even for unknown substances and look forward to support you as experts in facing the new challenges
With us, you are on the best way to assess the risks of your medical devices.