Design controls in the early development phase are essential for the development of a high-performance medical device. Design control is part of effective risk management to bring a safe product to market on time and within budget.
DESIGN CONTROL FOR LEGACY REMEDIATION AND NEW DEVELOPMENT PROJECTS
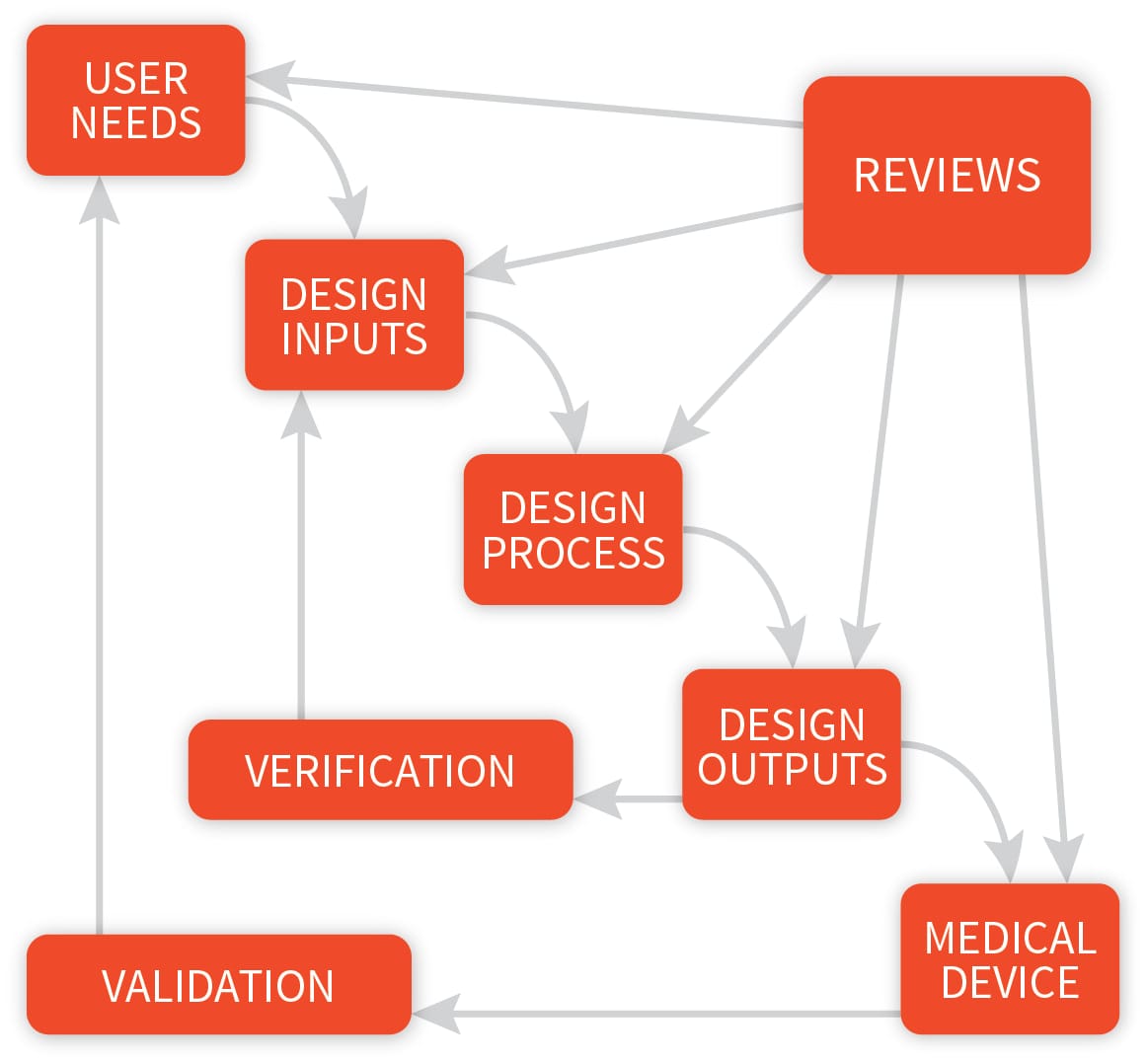
Design Controls need to be implemented from the early development stage. A proper design control process will not only assure compliance to FDA CFR 21 820.30 but also provide a tool and framework to develop a successful and well performing device.
We have huge experience in medical device field drafting design controls process and documentation for new device development or remediating existing Design History Files (DHF) to fill compliance gaps.
Our experience includes:
- Orthopedics and Osteosynthesis device
- Device incorporating software
- Endoscopic device
- Diagnostic devices
- Active devices
- Combination Products
DESIGN CONTROL IS A GOOD PRACTICE
In simple terms, it is the complete control over the design development of your product. Design Controls designates the application of a formal methodology to the conduct of product development activities.
Design controls make systematic assessment of the design an integral part of development. As a result, deficiencies in design input requirements, and discrepancies between the proposed designs and requirements, are made evident and corrected earlier in the development process.
Design controls increase the likelihood that the design transferred to production will translate into a device that is appropriate for its intended use.
In practice, design controls provide managers and designers with improved visibility of the design process. With improved visibility, managers are empowered to more effectively direct the design process—that is, to recognize problems earlier, make corrections, and adjust resource allocations.
Designers benefit both by enhanced understanding of the degree of conformance of a design to user and patient needs, and by improved communications and coordination among all participants in the process.
Design Controls – What are they?
A set of quality control practices and procedures incorporated into the design and development process
Control the design process to assure that device specifications meet:
Design Controls – Why?
It’s a beneficial business practice
- Systematic process
- Recognize problems early
- Make corrections
- Adjust resource allocation
- “Compliance at a Glance”
- Cost to correct design errors is lower when errors are detected early
- Project timelines
Design Controls are mandated by:
- 21CFR 820.30 in the US (Quality System Regulation)
- New MDR 2017/745
- ISO13485:2016 (Quality management systems) in Europe and around the world.
RISK MANAGEMENT FOR LEGACY AND NEW DEVELOPMENT PROJECTS
To fullfill the requirements of international standard and ensure your company gets a safe, effective product to market on time and within budget, you need a successful implementation of your risk management system. We can assist and help Your Company do it in the right way.
We offer the following services:
- Creation of risk file in compliance to ISO 14971:2019 for newly developed devices
- Remediation of existing risk management files (for medical devices and combination products)
- Gap Analysis services to check and improve the actual level of compliance of your risk management process to ISO 14971:2019
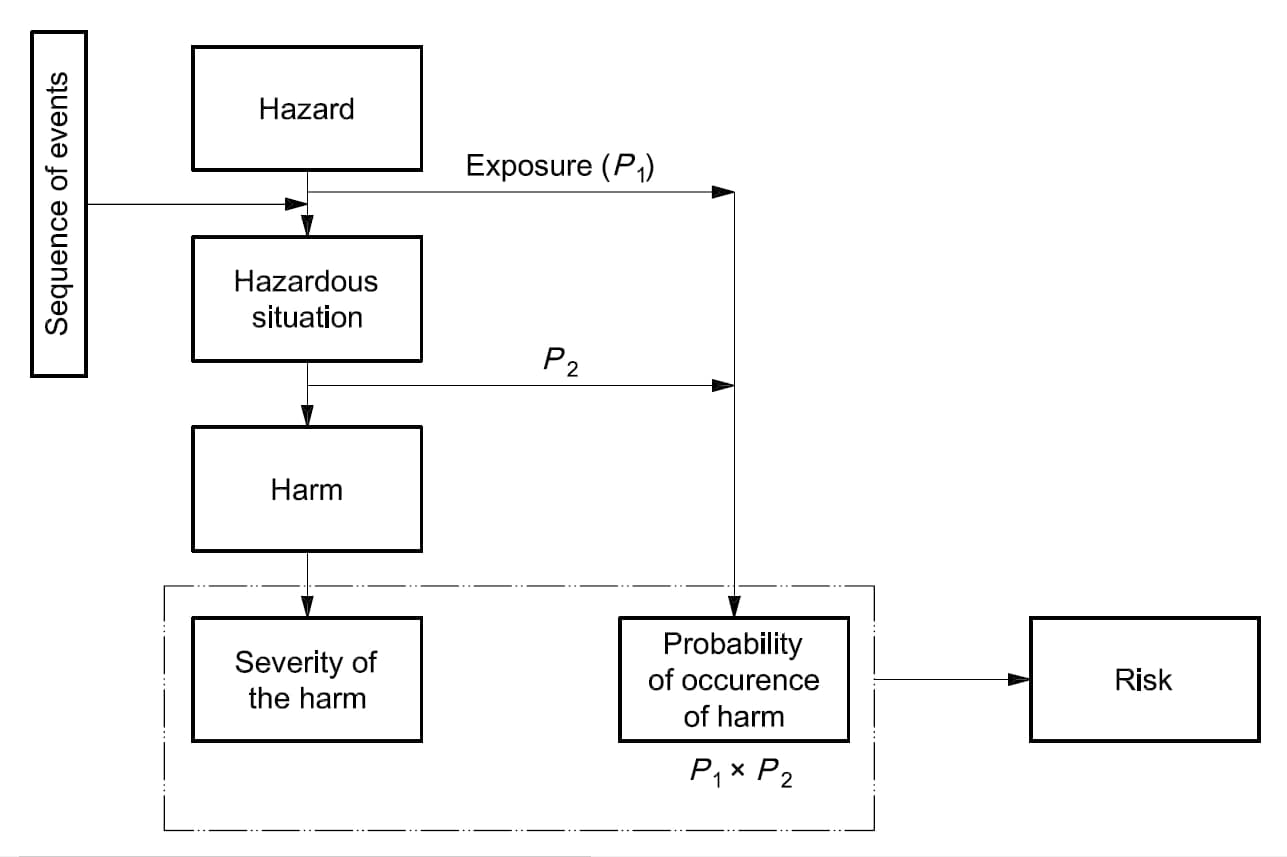
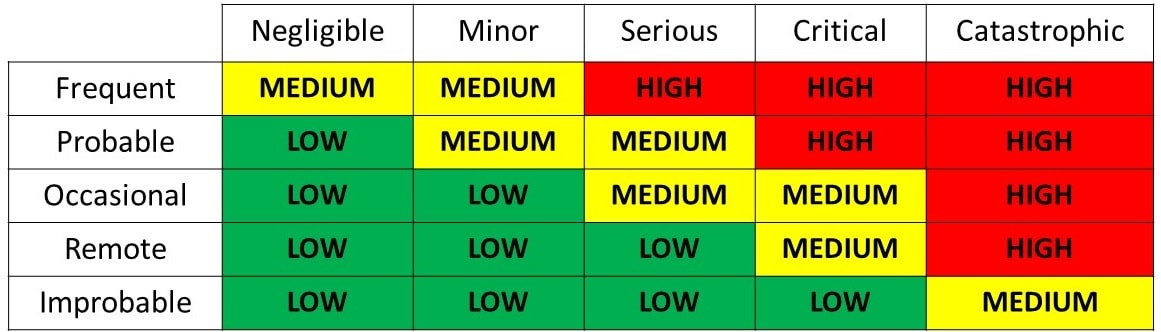
The requirements of ISO 14971:2019 are applicable to all stages of the life-cycle of a medical device.
Risk management is the systematic application of management policies, procedures and practices to the tasks of identifying, analysing, evaluating, controlling, monitoring and reviewing risk