The usage of CMR substances is strictly restricted and should be avoided whenever possible!
Our innovative and compact method enabling you to gain confidence about your materials meeting the requirements (in time and cost-effective manner.)
CMR substances are defined to be carcinogenic (C), mutagenic (M) and/or toxic to reproduction (R). They are grouped into different risk classes, in which class Ia and Ib substances possess the highest risk. As a consequence, these are regulated in particular by the Medical Devices Regulation.
Hence, their intended or non-intended addition should always be avoided and is part of the risk management. We look forward to support you in this process.
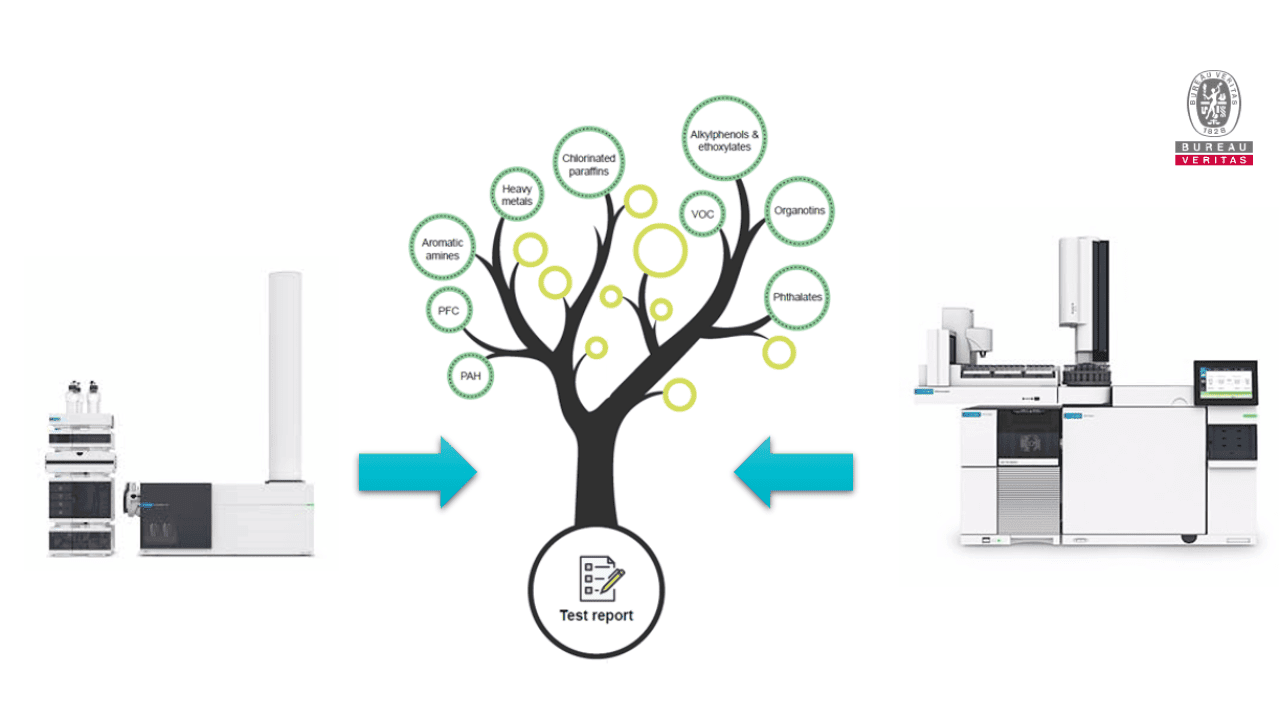
Bureau Veritas has developed a method that specifically searches for these substances. The innovative approach is based on a rapid screening method that first qualitatively determines the presence or absence of CMR substances. In case of findings, these are subsequently quantified by established analytical methods. This gives you an exact overview of the substances found and their concentration in your products.
Thus, it is possible for you to obtain a complete monitoring of your products. And this already in the development stage. This allows you to identify hazardous substances in various raw materials before they are integrated into your medical products. This saves resources - especially time and money.
If you would like to learn more about our complex and highly innovative method, please contact us.