CSEM has developed an optical method for measuring blood pressure: the revolutionary technology was commercialised as a medical product by the company Aktiia at the beginning of 2021.
High blood pressure - the dangerous widespread disease
High blood pressure is insidious because it is initially asymptomatic. Millions of people therefore have an increased risk of serious cardiovascular diseases due to their undetected high blood pressure. If at all, they control their blood pressure only sporadically and irregularly. One can certainly speak of high blood pressure as a "creeping killer".
Measuring blood pressure without a cuff - the revolution
Traditional blood pressure measurement has not changed in principle since it was invented over 100 years ago. The blood flow is gradually restricted via a cuff, and a change in the flow noise at certain cuff pressures defines upper and lower blood pressure values. But blood pressure can also be measured optically. This is the revolution. Until now, optical vascular monitoring only measured parameters such as partial oxygenation or heart rate. A closer look at the optical signals with high resolution, however, allows conclusions to be drawn about the pressure conditions in the vessel. CSEM has consistently pursued this approach and brought it to the product via the start-up AKTIIA.
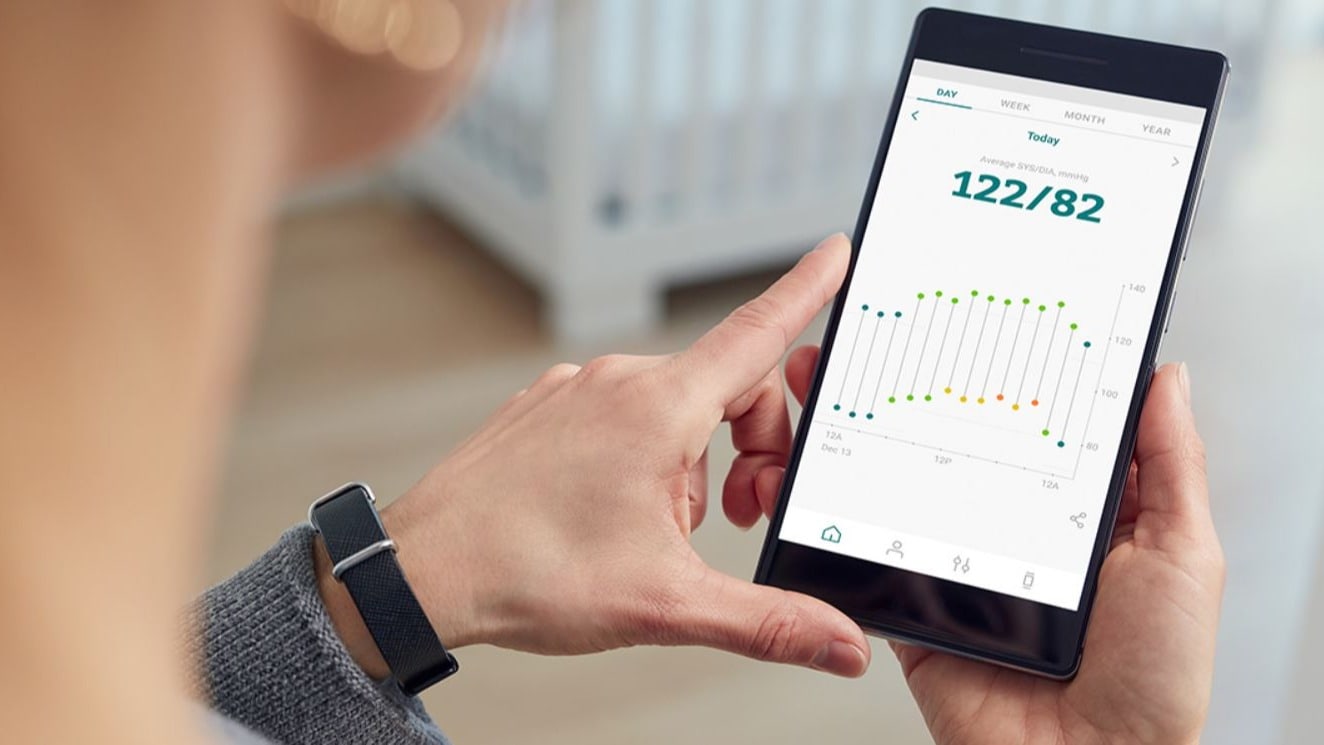
AKTIIA's system for optical blood pressure measurement
AKTIIA's solution for continuous, optical blood pressure measurement is a wearable that automatically measures the vascular dynamics of a heartbeat and derives the blood pressure from it. The wearer is not interrupted in his normal activities and is not subject to any measurement stress that could falsify the result. The values (about 100 measurements every week) can be read out conveniently via an app on the mobile phone and dangerous trends can be recognised in time. AKTIIA's wearable is CE certified and qualified as a class IIa medical device. It therefore meets the high quality and safety standards required for a product on the European market.
Background information
More information about Aktiia here.
Information about the certification here.