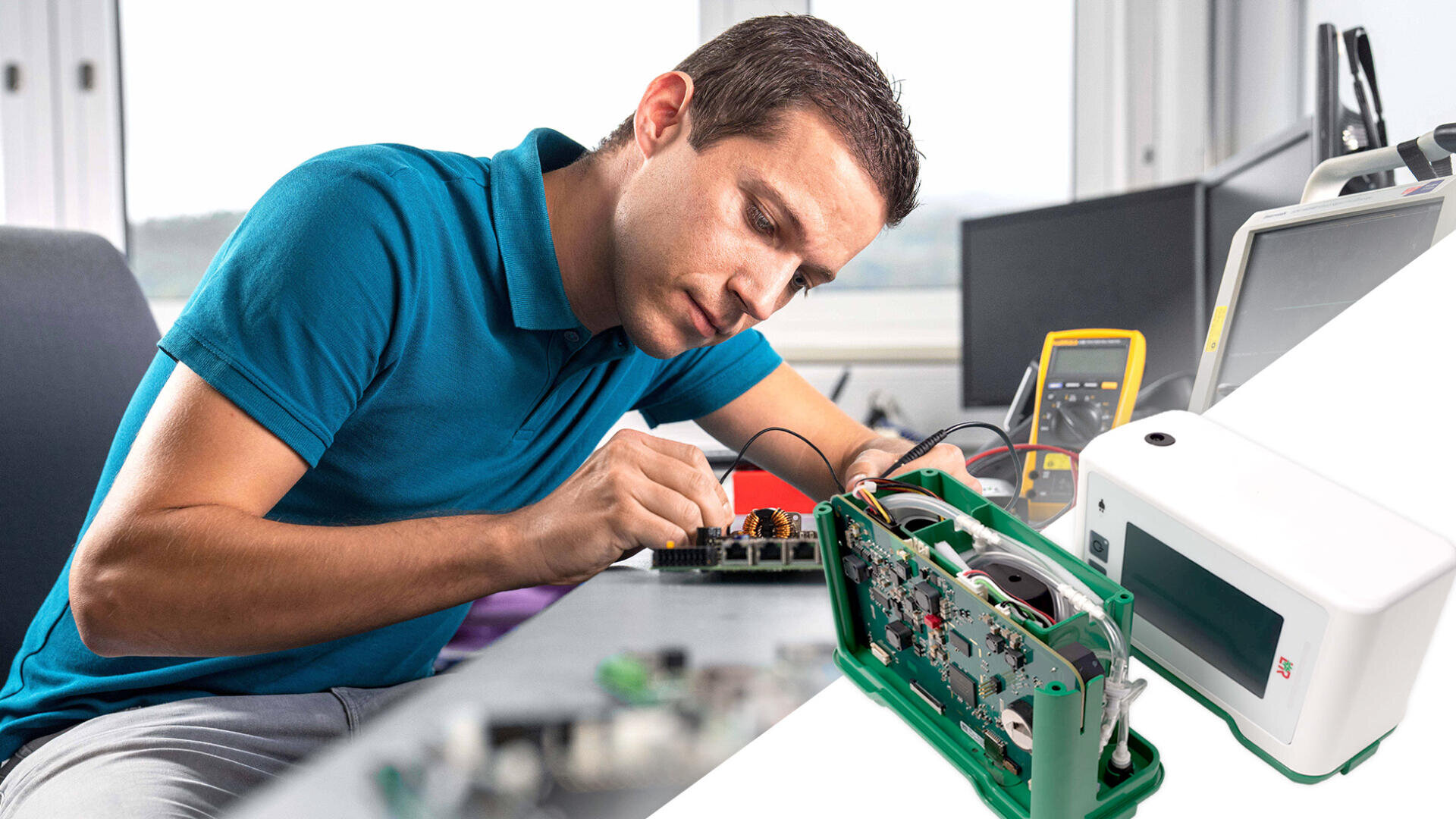
New electronics for a proven therapy device
Once again, our development team used its expertise to solve a problem in the field of medical technology. The electronics of the Suprasorb CNP P3 from the renowned manufacturer Lohmann & Rauscher (L&R) has been redesigned.
The Suprasorb CNP P3 is a negative pressure therapy device for wound care. It is established on the market, but various components that were previously used in it are no longer available. Therefore, a redesign of the electronics was carried out in order to secure production for the future. The customer also wanted a functional expansion as well as a facelift for the existing display.

Complex assignment
The challenge of redesigning the electronics proved to be a complex task: the device control software had to be able to run on both the previously used processor and the new processor. During the tight project period, the device also needed to undergo the required recertification. With the adjustments to the design and architecture, an additional abstraction layer was created for accessing the hardware drivers. CMake, an open-source/cross-platform build system, was used to compile and test both the previously used and the new software. In addition, due to the upgrade of the real-time operating system MQX from version 4 to version 5, the drivers required for the hardware, the pin multiplexing and the clock settings had to be adjusted. An additional abstraction layer was also introduced for the flash file system. The previous file system would not run on the new processor, so a new file system had to be evaluated and ported, and local adjustments made to the software.

Iftest redesigned the electronics of a negative pressure therapy device for wound treatment, securing and preparing production for the future.
Optimal coordination of software and hardware
In addition to a new microcontroller, new voltage converters, an acceleration sensor and a backlight driver were integrated on the hardware side. The dimensions were not allowed to change, as the device housing was to remain as it was. For the modified display, appropriate EMC filters were also provided on the RGB cables. In the in-house laboratory at Iftest AG, the efficacy of the filters was quickly verified in pre-compliance measurements.

An electronics redesign on every level: a redesign encompassing both hardware and software.
Individual solution for specific challenges
The prompt recertification made it possible for series production to start on time, as any interruption to the series-produced devices was impermissible for the end customer. It was therefore important to stick meticulously to the schedule. The new design allows the use of two different displays with the same housing. We were also able to develop a software version that runs on both processor types used. As a result, the desired functional expansion was achieved and the project was successfully completed.

After carrying out all desired adjustments and functional enhancements, the project was brought to a successful completion.
Added value in every respect
At the end of the day, it was worth the effort. We were able to create added value for our customer and optimally solve their problem of unavailable components. We would particularly like to thank the entire development and project teams.

Successfully revised negative pressure therapy device for wound treatment Suprasorb CNP P3 from Lohmann & Rauscher AG.
Have we piqued your interest?
If you have any questions about our engineering services and our other core competencies, Peter Traub looks forward to hearing from you.