Cleaning of 3D-printed devices
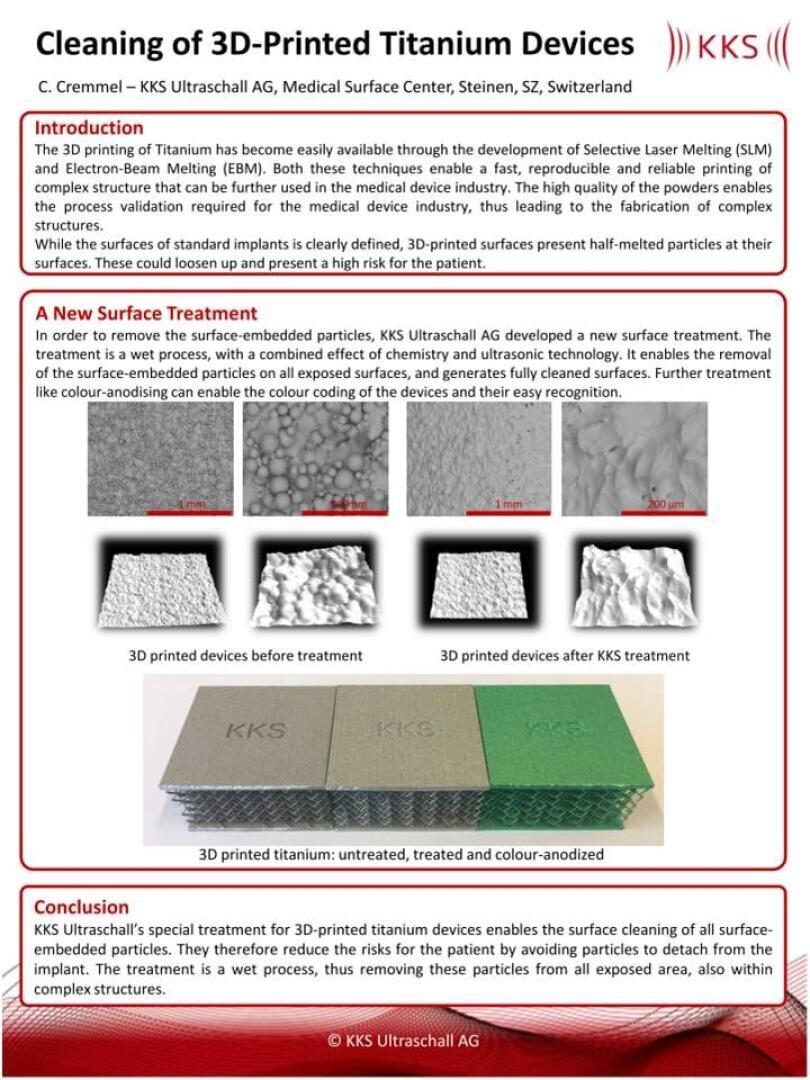
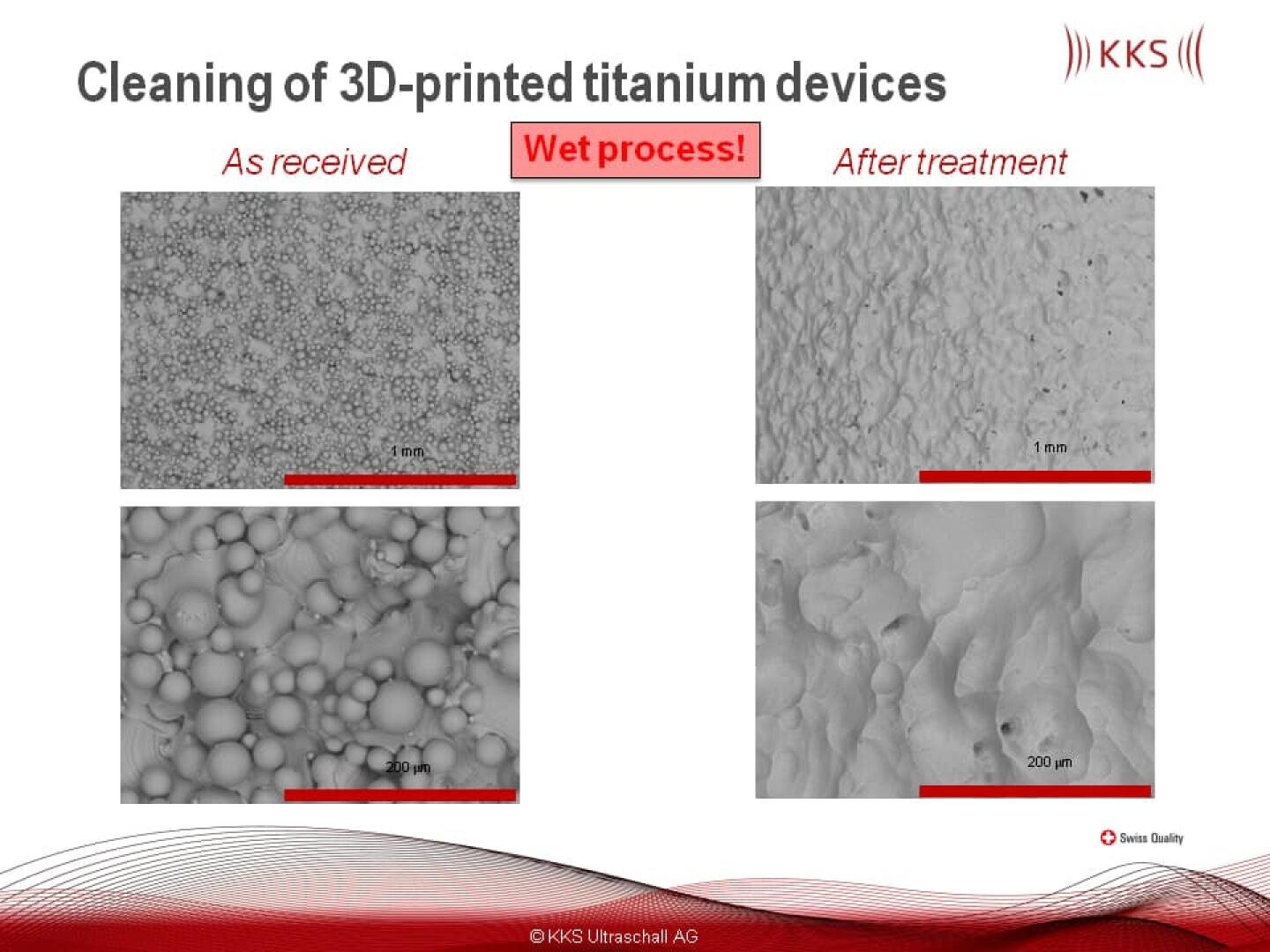
The actual issue with 3D-printed devices in the medical device industry is the presence of metal powder remains on the device itself. KKS developed a wet process for the removal of these particles.
KKS offers its long-lasting expertise and its passion for surface refinement to a worldwide customer base in the form of advanced services – services which we constantly adapt to changing needs while carrying forward our research into new and improved procedures.
In our Medical Surface Center in Switzerland, we collaborate with our customers to develop processes adapted to their actual preferences and continue our research on new and perfected methods.
Recently a new method for freeing 3-D printed devices of processing powders and contaminations was developed. The aim was to design a wet process, to enable the removal of processing powders in all pores or difficult-to-reach areas.
An example for such 3-D printed medical devices are grids, used as interbody cages for spine surgery. On such devices the surface quality is of highest importance as the bone has to grow back as fast as possible on the structure.